Why Do Noble Gases Have Similar Chemical Properties . on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. noble gases have uses that are derived from their other chemical properties. They show trends in their physical properties. The very low boiling points and melting points of the noble gases. they are known to possess extremely low chemical reactivity (hence the name inert gas). the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. This is because all the.
from www.slideserve.com
elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. they are known to possess extremely low chemical reactivity (hence the name inert gas). The very low boiling points and melting points of the noble gases. This is because all the. noble gases have uses that are derived from their other chemical properties. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. They show trends in their physical properties. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases.
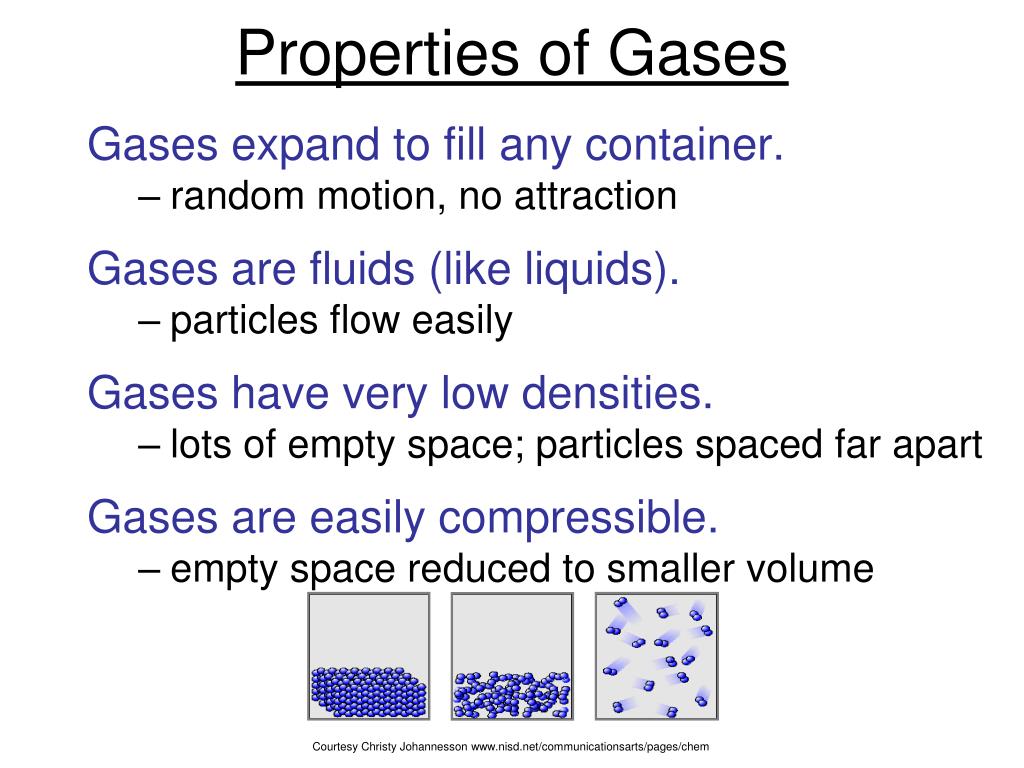
PPT Properties of Gases PowerPoint Presentation, free download ID
Why Do Noble Gases Have Similar Chemical Properties they are known to possess extremely low chemical reactivity (hence the name inert gas). elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. The very low boiling points and melting points of the noble gases. This is because all the. noble gases have uses that are derived from their other chemical properties. They show trends in their physical properties. they are known to possess extremely low chemical reactivity (hence the name inert gas).
From www.slideserve.com
PPT Group 18 Elements Noble Gases PowerPoint Presentation ID2168487 Why Do Noble Gases Have Similar Chemical Properties They show trends in their physical properties. they are known to possess extremely low chemical reactivity (hence the name inert gas). The very low boiling points and melting points of the noble gases. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. the. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideserve.com
PPT Properties of Gases PowerPoint Presentation, free download ID Why Do Noble Gases Have Similar Chemical Properties the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. This is because all the. The very low boiling points and melting points of the noble gases. They show trends in their physical properties. on the basis of experimental evidence relating. Why Do Noble Gases Have Similar Chemical Properties.
From warreninstitute.org
Exploring The Properties Of Noble Gases In Depth. Why Do Noble Gases Have Similar Chemical Properties noble gases have uses that are derived from their other chemical properties. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. They show trends in their physical properties. they are known to possess extremely low chemical reactivity (hence the name inert gas). The. Why Do Noble Gases Have Similar Chemical Properties.
From www.youtube.com
Noble Gases The Gases In Group 18 Properties of Matter Chemistry Why Do Noble Gases Have Similar Chemical Properties on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. This is because all the. The very low. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation ID149914 Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. noble gases have uses that are derived from their other chemical properties. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. They show trends in their physical properties.. Why Do Noble Gases Have Similar Chemical Properties.
From thechemistrynotes.com
Noble Gases Properties, Applications, Effects Why Do Noble Gases Have Similar Chemical Properties on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. They show trends in their physical properties. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. This is because all the. the noble gases (group. Why Do Noble Gases Have Similar Chemical Properties.
From www.vedantu.com
Facts About the Noble Gases Learn Important Terms and Concepts Why Do Noble Gases Have Similar Chemical Properties the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. They show trends in their physical properties. The very low boiling points and melting points of the noble gases. on the basis of experimental evidence relating chemical properties to electron distributions,. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Why Do Noble Gases Have Similar Chemical Properties elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. noble gases have uses that are derived from their other chemical properties. they are known to possess extremely low chemical reactivity (hence the name inert gas). They show trends in their physical properties. on the basis of experimental. Why Do Noble Gases Have Similar Chemical Properties.
From sciencenotes.org
What Are Noble Gases? Definition and Properties Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. they are known to possess extremely low chemical reactivity (hence the name inert gas). elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. the noble gases (group 18) are located in the far right of the. Why Do Noble Gases Have Similar Chemical Properties.
From www.pw.live
Chemical properties of noble gas Physics Wallah Why Do Noble Gases Have Similar Chemical Properties they are known to possess extremely low chemical reactivity (hence the name inert gas). They show trends in their physical properties. This is because all the. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. on the basis of experimental evidence relating chemical properties to electron distributions, it. Why Do Noble Gases Have Similar Chemical Properties.
From www.vedantu.com
Noble Gases Use and Its Properties Important Concepts and Tips for JEE Why Do Noble Gases Have Similar Chemical Properties They show trends in their physical properties. This is because all the. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. The. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideshare.net
Noble gases Why Do Noble Gases Have Similar Chemical Properties They show trends in their physical properties. This is because all the. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. . Why Do Noble Gases Have Similar Chemical Properties.
From sciencenotes.org
What Are Noble Gases? Definition and Properties Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. This is because all the. they are known to possess extremely low chemical reactivity (hence the name inert gas). on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. noble gases have. Why Do Noble Gases Have Similar Chemical Properties.
From owlcation.com
What's So Noble About Noble Gases? Owlcation Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. noble gases have uses that are derived from their other chemical properties. elements in the same group typically have similar chemical properties. Why Do Noble Gases Have Similar Chemical Properties.
From sciencequery.com
Noble gas definition and examples Why Do Noble Gases Have Similar Chemical Properties the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the. they are known to possess extremely low chemical reactivity (hence the name inert gas). This is because all the. They show trends in their physical properties. elements in the same. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideserve.com
PPT Group 18 Elements Noble Gases PowerPoint Presentation, free Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. They show trends in their. Why Do Noble Gases Have Similar Chemical Properties.
From www.slideserve.com
PPT Periodic Table of Elements PowerPoint Presentation, free download Why Do Noble Gases Have Similar Chemical Properties they are known to possess extremely low chemical reactivity (hence the name inert gas). They show trends in their physical properties. noble gases have uses that are derived from their other chemical properties. the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases. Why Do Noble Gases Have Similar Chemical Properties.
From pediaa.com
Difference Between Inert Gases and Noble Gases Definition, Properties Why Do Noble Gases Have Similar Chemical Properties The very low boiling points and melting points of the noble gases. on the basis of experimental evidence relating chemical properties to electron distributions, it was suggested that in the atoms of the noble gases. they are known to possess extremely low chemical reactivity (hence the name inert gas). the noble gases (group 18) are located in. Why Do Noble Gases Have Similar Chemical Properties.